Remember those high school chemistry classes where we were introduced to the fascinating world of gases? I can still picture myself struggling with those complex equations and trying to make sense of the relationships between pressure, volume, and temperature. Luckily, I had a helpful resource that guided me through it all: a gas laws worksheet. It wasn’t just a bunch of problems; it was a gateway to understanding the fundamental principles that govern the behavior of gases.

Image: classcampusbar.z19.web.core.windows.net
This blog post is dedicated to those of you who are seeking a clear and comprehensive understanding of gas laws. We’ll dive deep into the various gas laws, explore their historical development, and provide you with a free gas laws worksheet PDF complete with answers.
Exploring the Realm of Gas Laws
Gas laws are a set of fundamental principles that describe the relationship between the macroscopic properties of gases, namely pressure, volume, temperature, and the number of moles (amount). These laws have played a critical role in shaping our understanding of chemistry and physics, impacting fields such as meteorology, aviation, and even our daily lives.
The study of gas laws dates back to the 17th and 18th centuries, with scientists like Robert Boyle, Jacques Charles, and Joseph Gay-Lussac making groundbreaking discoveries. These pioneers meticulously observed the behavior of gases under different conditions, leading to the formulation of fundamental laws that now bear their names.
Unveiling the Key Gas Laws
Boyle’s Law
Boyle’s Law outlines the inverse relationship between the pressure and volume of a gas at constant temperature. This means that as the pressure on a gas increases, its volume decreases proportionally, and vice versa. The mathematical expression of Boyle’s Law is: P₁V₁ = P₂V₂.
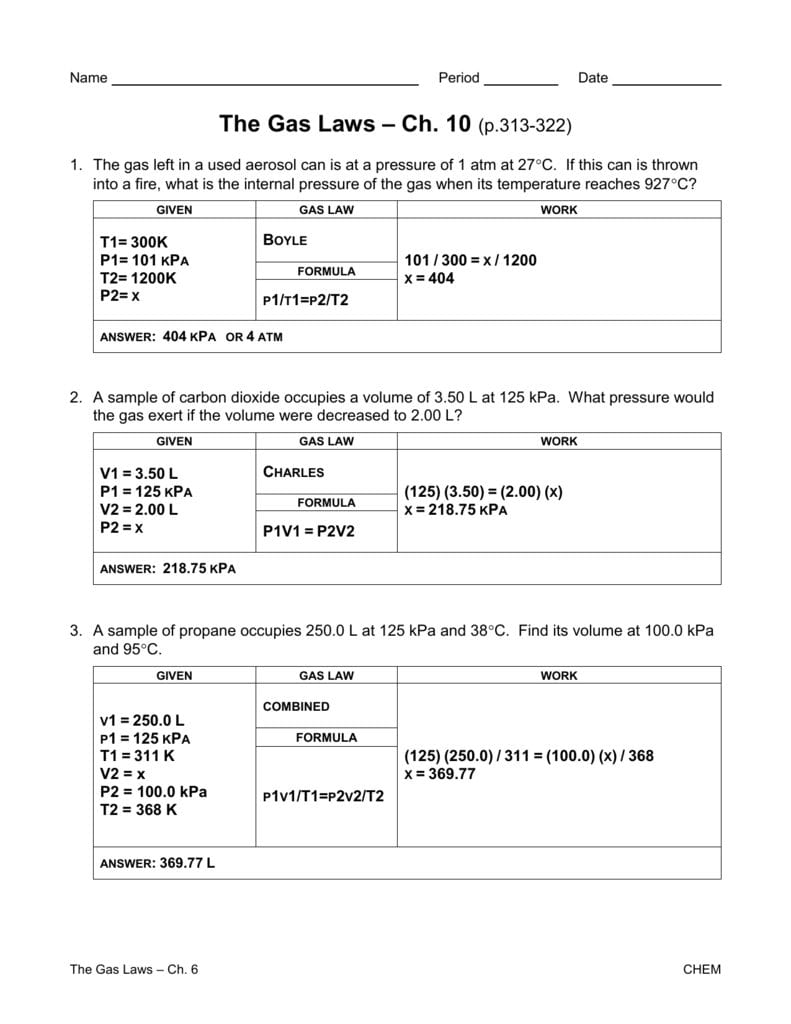
Image: materiallistmattie.z22.web.core.windows.net
Charles’s Law
Charles’s Law focuses on the direct relationship between the volume and temperature of a gas at constant pressure. This implies that as the temperature of a gas increases, its volume also increases proportionally, and vice versa, given constant pressure. The mathematical representation of Charles’s Law is: V₁/T₁ = V₂/T₂.
Gay-Lussac’s Law
Gay-Lussac’s Law describes the direct relationship between the pressure and temperature of a gas at constant volume. It states that as the temperature of a gas increases, its pressure increases proportionally, and vice versa, assuming a constant volume. This relationship is expressed mathematically as: P₁/T₁ = P₂/T₂.
Avogadro’s Law
Avogadro’s Law focuses on the relationship between the volume and number of moles of a gas at constant temperature and pressure. It establishes that equal volumes of any ideal gas, under the same conditions of temperature and pressure, contain the same number of molecules. This is expressed as: V₁/n₁ = V₂/n₂.
The Ideal Gas Law
The Ideal Gas Law combines all the aforementioned laws into a single, comprehensive equation that describes the behavior of ideal gases. The Ideal Gas Law is given by: PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
The Importance of Understanding Gas Laws
Understanding gas laws is crucial in various fields, from engineering and chemistry to meteorology and aviation. Here are some noteworthy applications:
- Meteorology: Weather forecasting heavily relies on gas laws to understand changes in air pressure, temperature, and volume, influencing atmospheric conditions.
- Aviation: The behavior of gases in aircraft engines is determined by gas laws, enabling efficient flight operations and fuel consumption.
- Chemistry: Gas laws are fundamental in chemical reactions, allowing scientists to predict and manipulate the behavior of gases involved in reactions.
- Medical Field: Gas laws play a role in understanding the function of the lungs, respiration, and the delivery of oxygen to our bodies.
Gas Laws Worksheet PDF with Answers: Your Learning Companion
To help you solidify your understanding of the gas laws, we’ve created a comprehensive Gas Laws Worksheet PDF with Answers. This worksheet incorporates a variety of problems ranging from basic applications to more complex scenarios, challenging you to apply the gas laws in practical situations.
The worksheet covers topics such as:
- Converting between units of pressure, volume, and temperature
- Applying the Ideal Gas Law to calculate unknown variables
- Solving problems involving gas mixtures
- Analyzing real-world scenarios involving gas laws
Tips for Mastering Gas Laws
When tackling gas law problems, consider these tips:
- Understand the Concepts: Ensure a clear understanding of each gas law and its underlying principles before solving problems.
- Unit Consistency: Always ensure that all units used in your calculations are consistent, converting to the same units when necessary.
- Step-by-Step Approach: Break down complex problems into smaller, manageable steps to avoid confusion and errors.
- Practice Regularly: The key to mastering gas laws is through consistent practice. Work through numerous problems to strengthen your understanding and problem-solving skills.
Expert Advice
As an experienced chemist and educator, I’ve learned that one of the most effective methods for mastering gas laws is to visualize the concepts. Draw diagrams representing the relationships between pressure, volume, temperature, and number of moles. This visualization can enhance comprehension and aid in problem-solving.
Another piece of advice is to stay curious. Gas laws are a fascinating aspect of chemistry, so explore real-world applications and delve deeper into the historical context behind these laws. The more you learn, the better you’ll grasp the significance and practical implications of gas laws.
FAQ on Gas Laws:
Here are some frequently asked questions about gas laws:
Q: What is an ideal gas?
A: An ideal gas is a theoretical concept that assumes that gas molecules have no volume and do not interact with each other. While real gases deviate from this ideal behavior, the ideal gas model provides a useful approximation for many practical situations.
Q: Why are gas laws important in our daily lives?
A: Gas laws underpin many aspects of our daily life, including breathing, cooking, and transportation. They are essential for understanding weather patterns, operating engines, and even controlling the pressure in our tires.
Q: What are some common examples of gas laws in action?
A: Some everyday examples include: the pressure buildup in a sealed container when heated, the expansion of hot air balloons, and the deflation of a tire in cold weather.
Gas Laws Worksheet Pdf With Answers
https://youtube.com/watch?v=Esjo0Ou_4gE
Conclusion
In conclusion, understanding gas laws is essential for anyone pursuing studies in science, engineering, or any field dealing with the behavior of gases. By mastering these fundamental principles, you can gain a deeper appreciation for the world around us and effectively tackle practical problems relating to gas behavior.
Are you interested in learning more about gas laws and exploring the fascinating world of chemistry? Let us know in the comments below!






