Have you ever wondered why sugar dissolves in water, but eventually, you can’t add any more? Or why salt seems to disappear in water, but you can keep adding more and more? These are examples of saturated and unsaturated solutions, respectively. Understanding these concepts is essential for comprehending the fundamentals of chemistry, and it’s a common topic explored in school science classes, often through worksheets. In this guide, we’ll delve into the fascinating world of solutions, exploring the differences between saturated and unsaturated solutions, and provide you with helpful tips for completing your “saturated and unsaturated solutions worksheet answers” with confidence.
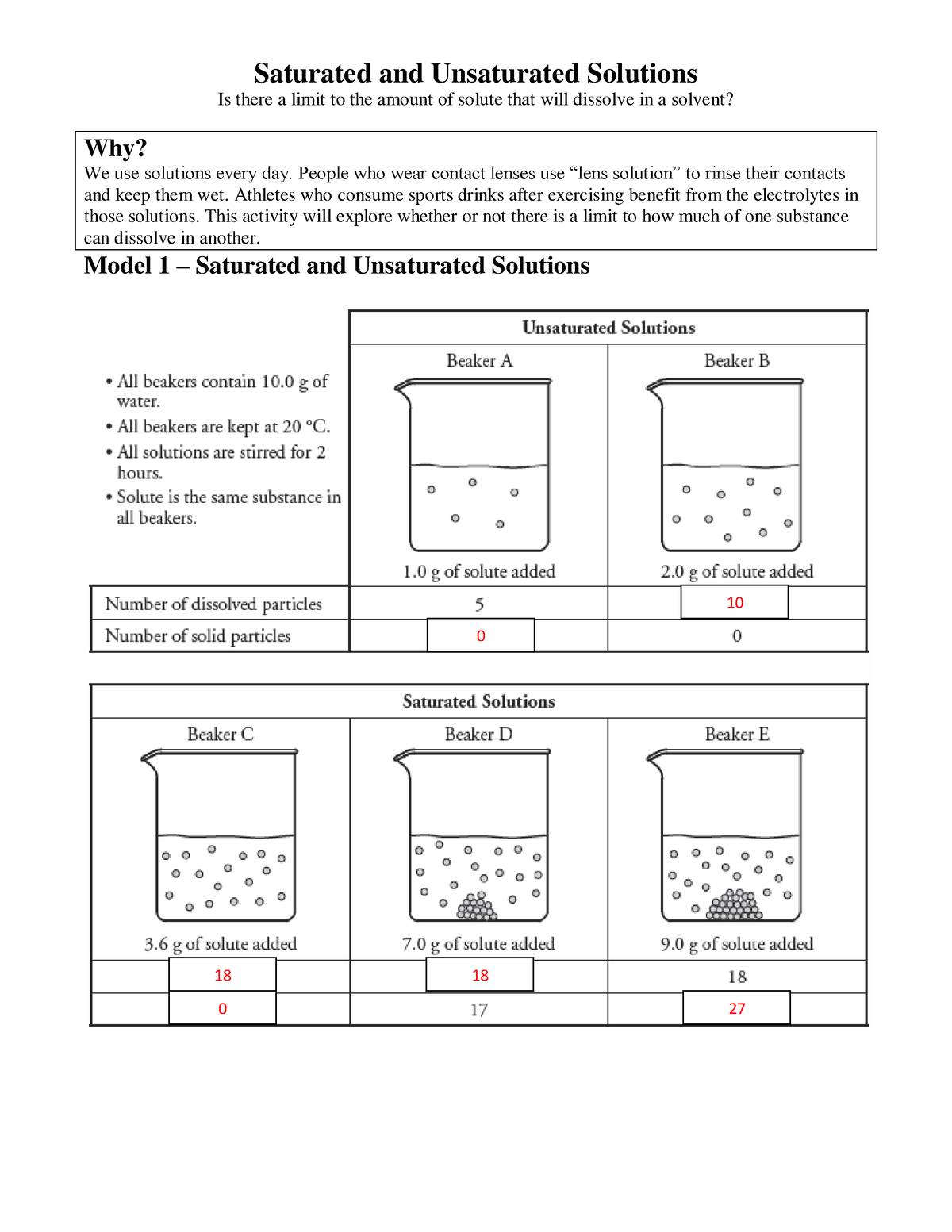
Image: www.studocu.com
Imagine you’re making a cup of tea. You add a teaspoon of sugar and stir until it disappears. You add another, and another, and eventually, you reach a point where no more sugar seems to dissolve, and it starts to settle at the bottom. That’s a sign that you’ve reached a “saturation point,” where the solution can’t hold any more solute. This is similar to how you can’t add more people to a crowded room without someone standing outside. This is where the concept of solution saturation comes into play – the ability of a solvent to dissolve a certain amount of solute at a specific temperature.
Saturated and Unsaturated Solutions
A solution is a homogeneous mixture where one substance, called the solute, dissolves in another substance, called the solvent. The concentration of a solution refers to how much solute is dissolved in a given amount of solvent. Solutions can be categorized into three types: saturated, unsaturated, and supersaturated.
Saturated Solutions
A saturated solution is a solution that contains the maximum amount of solute that can be dissolved at a given temperature and pressure. If you try to add more solute to a saturated solution, it will not dissolve and will simply settle to the bottom as a precipitate. In our tea example, the solution reached saturation when the sugar stopped dissolving and started accumulating at the bottom of the cup.
Unsaturated Solutions
An unsaturated solution is a solution that contains less solute than the maximum amount that can be dissolved at a given temperature and pressure. In other words, it has the capacity to dissolve more solute. For instance, if you add a small amount of sugar to your tea, it will dissolve completely. You can continue adding more sugar until it reaches the saturation point. The solution remains unsaturated until you reach the maximum capacity of dissolved solute.

Image: davida.davivienda.com
Supersaturated Solutions
A supersaturated solution is a solution that contains more solute than the maximum amount that can be dissolved at a given temperature and pressure. These solutions are unstable and can be created by carefully dissolving a large amount of solute in a solvent at a high temperature, then slowly cooling the solution. Upon cooling, the excess solute may remain dissolved for a while, but any disturbance, like adding a seed crystal, can cause the excess solute to precipitate out of the solution. Supersaturated solutions are essentially a “balancing act” where the conditions are just right to keep the excess solute dissolved. Even the slightest change can tip the scales and cause the excess solute to crystallize out.
Factors Affecting Saturation
Several factors can affect the saturation point of a solution, including:
- **Temperature:** Increasing the temperature generally increases the solubility of most solids in liquids. As temperature increases, the solvent molecules have more kinetic energy, allowing them to break apart the solute molecules more effectively.
- **Pressure:** Pressure has a significant impact on the solubility of gases in liquids. As pressure increases, the solubility of a gas increases. This is why fizzy drinks are bottled under high pressure, to dissolve more carbon dioxide. When you open the bottle, the pressure decreases, and the gas bubbles escape, leading to fizz.
- **Nature of the solute and solvent:** The type of solute and solvent play a crucial role in determining solubility. For example, ionic compounds like salt are generally soluble in polar solvents like water, while nonpolar compounds like oil are generally soluble in nonpolar solvents like hexane. Similar molecules attract each other (“like dissolves like”).
Solving Saturated and Unsaturated Solutions Worksheets
Saturated and unsaturated solutions worksheets are designed to test your understanding of the concepts we’ve discussed. They will often present you with scenarios involving solutions and ask you to determine whether a solution is saturated, unsaturated, or supersaturated, or to predict how much solute can be dissolved. Here are some tips to help you solve these worksheets effectively:
Tips for Solving Worksheets
- **Understand the key definitions:** Make sure you clearly understand the definitions of saturated, unsaturated, and supersaturated solutions.
- **Identify the solute and solvent:** Clearly identify the solute and solvent in each scenario.
- **Consider the temperature and pressure:** If the worksheet provides information about temperature and pressure, keep in mind how these factors can affect solubility.
- **Refer to a solubility chart:** If necessary, consult a solubility chart for the specific solute and solvent involved in the problem to determine the maximum solubility at a given temperature.
- **Practice calculations:** If the worksheet requires you to calculate the amount of solute that can be dissolved, practice using solubility formulas and converting between units.
- **Visualize the scenario:** Sometimes, visualizing the scenario can help you understand the concept. Imagine the molecules interacting and the solute dissolving in the solvent. If you can imagine what’s happening at the molecular level, it will make the concepts easier to grasp.
FAQs about Saturated and Unsaturated Solutions
Here are some frequently asked questions about saturated and unsaturated solutions:
What happens if you try to dissolve more solute in a saturated solution?
If you try to dissolve more solute in a saturated solution, it will not dissolve. Instead, it will settle to the bottom of the container as a precipitate. This indicates that the solution has reached its maximum capacity for dissolved solute.
How can you make a supersaturated solution?
Supersaturated solutions can be made by heating a solution and dissolving more solute than its normal solubility at that temperature. This creates an unstable state where the excess solute remains dissolved. If you allow the solution to cool slowly, the excess solute may continue to stay dissolved, making it supersaturated. However, any disturbance, like adding a seed crystal, can trigger the excess solute to crystallize out.
What are the practical applications of saturated and unsaturated solutions?
Saturated and unsaturated solutions have numerous practical applications in various scientific fields and everyday life. Some examples include:
- Crystallization: The process of making crystals, like sugar crystals or salt crystals, involves the creation and manipulation of saturated and supersaturated solutions.
- Medicine: Many medicines are solutions that must be carefully prepared at specific concentrations to ensure the most effective dosage and minimal side effects.
- Agriculture: Farmers use solutions of nutrients to fertilize crops, and the concentration of these solutions must be carefully controlled to ensure the plants receive the optimal amount of nutrients without causing damage.
- Food production: Sugar and salt solutions are used extensively in food preservation, flavoring, and creating desirable textures, and the saturation of these solutions impacts their effectiveness.
- Environmental science: The understanding of saturation in water bodies plays a crucial role in environmental science, as it affects the ability of water to hold dissolved substances, such as pollutants, and affects the health of aquatic ecosystems.
Saturated And Unsaturated Solutions Worksheet Answers
Conclusion
Saturated and unsaturated solutions are essential concepts in chemistry that have wide-ranging applications. Being able to identify and understand the properties of these solutions is necessary for solving problems and performing laboratory experiments. Remember to practice, refer to solubility charts when necessary, and most importantly, visualize the scenarios to enhance your comprehension. By mastering these concepts, you’ll be well-equipped to tackle any “saturated and unsaturated solutions worksheet answers” that come your way. Do you often struggle with these concepts? Are you confident in your understanding? Let us know in the comments below!






