Remember that chemistry experiment in high school where you carefully dripped a solution from a burette into a flask, watching the color change with bated breath? That was titration, a fundamental technique in chemistry used to determine the concentration of an unknown solution—a vital skill for anyone venturing into the world of chemical analysis. Titration is a bit like baking a cake, where you add ingredients (solutions) in precise amounts to achieve a specific outcome. In this case, we’re combining acids and bases in controlled reactions to calculate the unknown concentration.

Image: woodsholemuseum.org
From determining the acidity of your stomach to figuring out the purity of your household cleaning products, titration has countless applications across various fields, including pharmaceuticals, agriculture, and environmental monitoring. This detailed guide delves into the intricacies of titration, helping you understand the process, interpret results, and write a perfect lab report.
Understanding Titration: The Art of Chemical Precision
Titration is a quantitative chemical analysis method where a solution of known concentration, called the titrant, is gradually added to a solution of unknown concentration, called the analyte. The reaction between the titrant and analyte is usually a neutralization reaction, where an acid reacts with a base.
The key to titration is the equivalence point, the point at which the reactants have completely reacted. This point is usually indicated by a color change, often achieved using an indicator. Indicators are substances that change color depending on the pH of the solution, helping us pinpoint the equivalence point. Think of them as tiny chemical traffic lights, signaling when the reaction is complete.
Types of Titration: Delving into the Variations
Acid-Base Titration:
This is the most common type of titration where an acid is neutralized by a base, or vice versa. An example is titrating a solution of unknown concentration of a strong acid, like hydrochloric acid (HCl), with a solution of known concentration of a strong base, like sodium hydroxide (NaOH). The reaction produces salt and water.
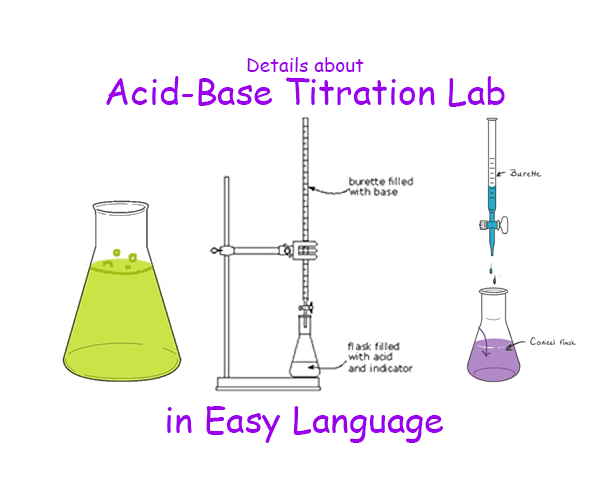
Image: tuitiontube.com
Redox Titration:
This involves a reaction between an oxidizing agent and a reducing agent. Here, the titrant is either an oxidizing or reducing agent, and the analyte is a reducing or oxidizing agent, respectively. A classic example is titrating potassium permanganate (KMnO4), a strong oxidizing agent, with iron(II) ions (Fe2+), a reducing agent.
Precipitation Titration:
This type of titration involves a reaction that results in the formation of a precipitate, an insoluble solid. For example, titrating a solution of silver nitrate (AgNO3) with a solution of sodium chloride (NaCl) will produce a white precipitate of silver chloride (AgCl).
Titration Procedure: A Step-by-Step Guide
Performing a titration is a precise and meticulous process that requires careful attention to detail. Here’s a breakdown of the common steps involved:
- Prepare the Analyte Solution: Accurately weigh or measure a known volume of the analyte solution and carefully transfer it to a clean flask or beaker.
- Add Indicator: Add a few drops of an appropriate indicator to the analyte solution. The indicator will change color at the equivalence point, indicating the end of the reaction.
- Fill the Burette: Clean and fill the burette with the titrant solution. Ensure that the burette is properly calibrated and that the air bubbles are removed before starting the titration.
- Titrate Gradually: Slowly add the titrant from the burette to the analyte solution, stirring constantly. Keep your eye on the color change.
- Note the Equivalence Point: Once the color change is observed and persists for at least 30 seconds, stop the titration and record the volume of titrant used.
- Repeat the Titration: Perform the titration at least three times to ensure accuracy and consistency in the results.
Analyzing the Data: Getting to the Heart of the Matter
Once you’ve completed the titration and collected your data, the next step is to analyze the results to determine the unknown concentration of the analyte. This involves a series of calculations and is best explained with an example.
Example: Determining the Concentration of an Unknown Acid
Let’s say you titrated 25.00 mL of an unknown concentration of hydrochloric acid (HCl) with 0.100 M sodium hydroxide (NaOH). The volume of NaOH used to reach the equivalence point was 20.00 mL.
1. **Write the Balanced Chemical Equation:**
HCl (aq) + NaOH (aq) –> NaCl (aq) + H2O (l)
2. **Calculate the Moles of NaOH:**
Moles of NaOH = (Concentration of NaOH) x (Volume of NaOH)
= (0.100 mol/L) x (0.0200 L)
= 0.00200 mol
3. **Determine the Moles of HCl:**
From the balanced equation, we know that 1 mole of HCl reacts with 1 mole of NaOH.
Moles of HCl = Moles of NaOH = 0.00200 mol
4. **Calculate the Concentration of HCl:**
Concentration of HCl = (Moles of HCl) / (Volume of HCl)
= 0.00200 mol / 0.0250 L
= 0.0800 M
Therefore, the concentration of the unknown HCl solution is 0.0800 M.
Tips and Expert Advice for Mastering Titration
Titration is a technique that requires practice and precision. Here are some tips from my experience to improve your titration skills:
- Use High-Quality Chemicals: Choose analytical-grade reagents for more accurate results.
- Ensure Proper Burette Calibration: Calibrate the burette before each experiment to ensure accurate volume measurements.
- Read the Burette Carefully: Make sure to read the volume markings on the burette accurately, especially near the meniscus.
- Stir the Solution Constantly: Continuous stirring helps ensure that the analyte solution is homogeneous and that the titrant is evenly distributed.
- Choose the Right Indicator: Select an indicator that changes color near the equivalence point of the reaction.
- Practice Makes Perfect: Just like any skill, titration requires practice to master. The more titrations you perform, the better you’ll become at finding the equivalence point and interpreting the results.
Frequently Asked Questions (FAQs)
Q: What are some common sources of error in titration?
A: Common sources of error include inaccurate burette readings, incorrect calibration, incomplete reaction, using a wrong indicator, contamination of solutions, and improper cleaning of glassware.
Q: How do I choose the right indicator for my titration?
A: You should choose an indicator that changes color near the equivalence point of the reaction. The indicator should also be compatible with the analyte solution. Refer to an indicator chart for guidance.
Q: What is the difference between the equivalence point and the endpoint?
A: The equivalence point is the theoretical point where the acid and base have completely neutralized each other. The endpoint is the point where the indicator changes color, signaling the end of the reaction. While these points should ideally be the same, there can be slight discrepancies depending on the indicator used and the reaction.
Q: What are some real-world applications of titration?
A: Titration has numerous applications in various fields, including:
- Pharmaceuticals: determining the purity and potency of drugs and medications
- Agriculture: analyzing soil and fertilizers
- Environmental monitoring: determining the concentration of pollutants in water or air
- Food industry: checking the acidity of food products, such as vinegar or juice
- Chemical manufacturing: controlling the quality of chemical products
Lab Report For Titration Of Acids And Bases
Conclusion
Titration is a versatile and crucial analytical technique with wide-ranging applications in chemistry and related fields. By understanding the principles, procedures, and data analysis involved in titration, you can effectively determine the concentration of unknown solutions and contribute valuable insights to scientific research, industrial processes, and everyday life.
Are you familiar with titration and its applications in your field of study or work? Share your thoughts and experiences in the comments below!






