Have you ever wondered what makes those sweet smells of ripe fruits or the pungent aroma of cinnamon so captivating? The answer lies in the fascinating world of organic chemistry, specifically in the realm of aldehydes and ketones. These seemingly simple molecules, with their unique structures and reactivity, are the architects behind a vast array of scents, flavors, and even essential biological functions.
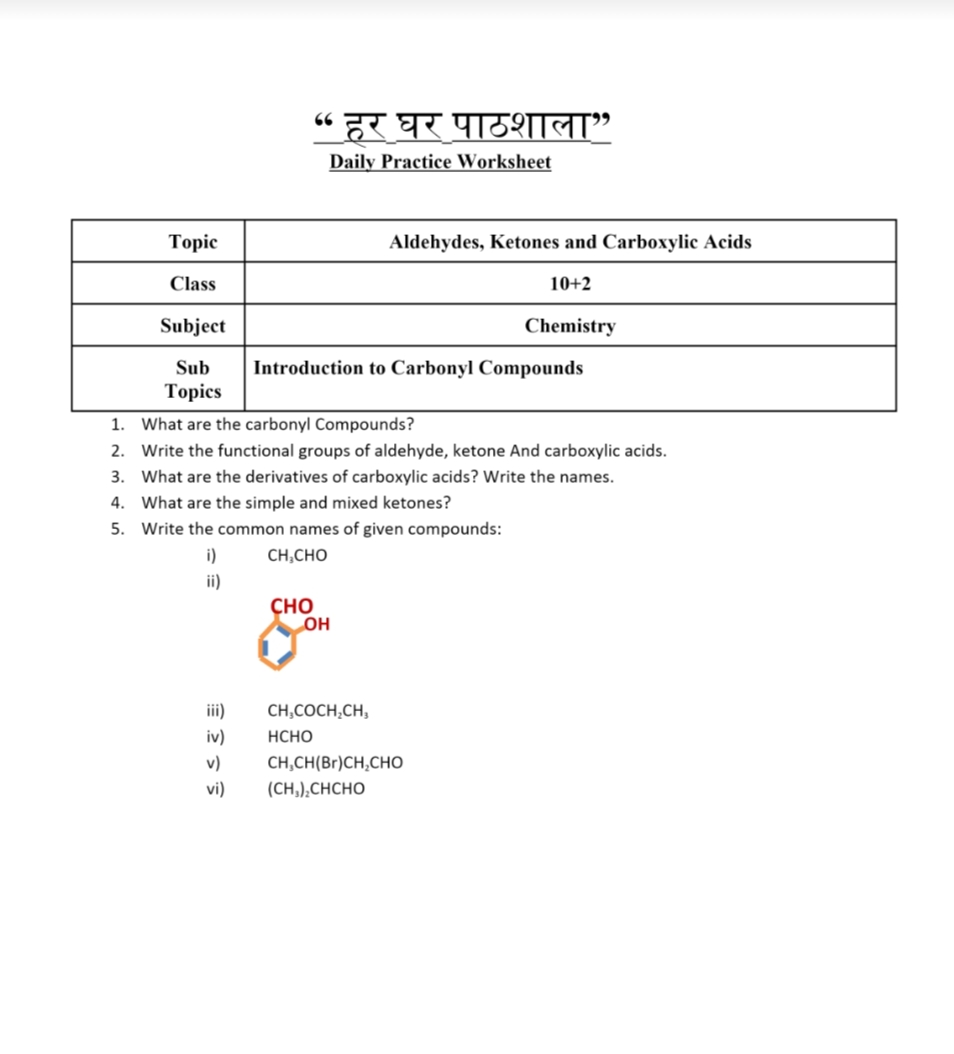
Image: www.teachmint.com
This lab report journey embarks on an exploration of the properties of aldehydes and ketones, delving into their intricate structures, distinct chemical reactions, and practical applications. We’ll unravel the mysteries of these remarkable compounds, examining their impact on our everyday lives from the fragrances we wear to the medications we rely on.
Delving into the Heart of Aldehydes and Ketones
Aldehydes and ketones are organic compounds characterized by a carbonyl group, a carbon atom double-bonded to an oxygen atom (C=O). The key difference between them lies in the arrangement of atoms attached to this carbonyl group. In aldehydes, the carbonyl group is bonded to at least one hydrogen atom, while in ketones, it is attached to two carbon atoms.
This seemingly subtle difference has profound implications for their chemical properties and reactivity. Aldehydes, with their lone hydrogen atom, exhibit greater reactivity, readily participating in oxidation-reduction reactions, a phenomenon that plays a crucial role in biological processes. Ketones, on the other hand, are more stable due to the absence of this reactive hydrogen, lending them stability in various chemical environments.
Unmasking the Properties Through Experiments
Our lab journey delves into the diverse properties of aldehydes and ketones through a series of carefully designed experiments. Each experiment serves as a window into the unique characteristics of these compounds, unveiling insights into their reactivity, interactions, and applications.
**1. The Tale of Tollen’s Test:**
A classic chemical test, Tollen’s test, serves as a powerful tool for identifying aldehydes. This reaction relies on the ability of aldehydes, but not ketones, to reduce silver ions in a silver ammonia complex to metallic silver. Visually, this reaction manifests as a beautiful silver mirror coating the inside of the reaction vessel, a testament to the remarkable oxidizing power of aldehydes.
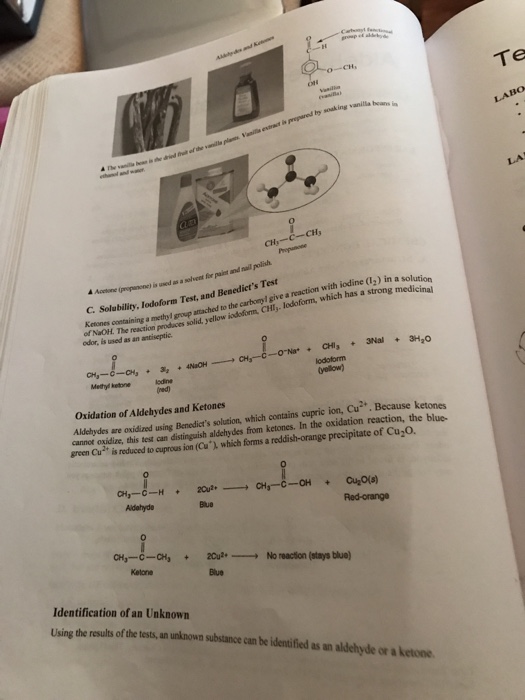
Image: www.chegg.com
**2. The Iodoform Test: Unmasking the Presence of Methyl Ketones:**
The Iodoform test, meanwhile, offers a distinctive way to identify methyl ketones. In this reaction, the methyl ketone reacts with iodine in a basic solution, resulting in the formation of a yellow precipitate of iodoform. This characteristic yellow solid unambiguously reveals the presence of a methyl ketone in the sample.
**3. The Benedicts Test: Unveiling Reducing Sugars:**
Moving beyond the realm of simple aldehydes and ketones, we venture into the world of carbohydrates, specifically those containing reducing sugars. The Benedicts test utilizes the reducing properties of aldehydes present in these sugars. The telltale sign of a positive result is the formation of a brick-red precipitate, signifying the presence of a reducing sugar, a key component in various biological processes.
**4. The Schiff’s Test: An Exploration of Aldehyde Reactivity:**
Schiff’s test offers a unique perspective on the reactivity of aldehydes. In this reaction, an aldehyde reacts with a colorless Schiff’s reagent, resulting in a vibrant purple coloration. This color change provides a clear-cut indication of the presence of an aldehyde, confirming its capacity to interact with the reagent.
**5. Exploring the Reactivity of Aldehydes and Ketones:**
Beyond these specific tests, we delve deeper into the general reactivity of aldehydes and ketones. Through a series of carefully designed experiments, we explore their reactions with various reagents, including grignard reagents, wittig reagents, and various oxidizing and reducing agents. These experiments shed light on the diverse pathways these molecules can traverse, highlighting their versatility in organic synthesis.
The World Beyond the Lab: Real-World Applications
Aldehydes and ketones are not merely confined to the realm of academic experiments. They play a vital role in our everyday lives, shaping scents, flavors, and even the very essence of life itself.
**1. The Sweet Embrace of Flavors:**
Think of the alluring aroma of vanilla extract, the sweet scent of cinnamon, or the tangy taste of citrus fruits. All of these familiar and beloved flavors are derived from the presence of aldehydes and ketones. These compounds contribute to the complex tapestry of tastes we experience, adding vibrancy and depth to our culinary landscape.
**2. The Art of Perfumery:**
The captivating world of perfumery is intricately intertwined with aldehydes and ketones. These compounds, with their diverse fragrances, serve as the building blocks for an astonishing array of perfumes, from delicate floral scents to robust woody aromas. Aldehydes, in particular, lend a distinct crispness and freshness to fragrances.
**3. The Chemistry of Life:**
Aldehydes and ketones are not just mere ingredients in our daily lives; they are fundamental building blocks of life itself. Glucose, the primary energy source for our bodies, is an aldose, an aldehyde sugar. Ketones are also integral to the workings of our bodies, playing crucial roles in metabolic pathways and serving as signaling molecules.
**4. The Role in Pharmaceuticals:**
Aldehydes and ketones are also vital components of many pharmaceutical compounds. From pain relievers and antiseptics to antifungal agents, these compounds find their place in a wide spectrum of medications, contributing to our well-being.
Mastering the Secrets: Expert Insights and Actionable Tips
The knowledge gained through these experiments and observations empowers us to understand and leverage the properties of aldehydes and ketones in various applications. Here are some key insights and actionable tips drawn from expert knowledge:
- Safety first: Always handle aldehydes and ketones with caution, as they can be flammable and irritating. Work in a well-ventilated area and wear appropriate protective gear like gloves and goggles.
- Storage matters: Store aldehydes and ketones in sealed containers away from heat and direct sunlight to prevent degradation and potential hazards.
- The power of careful observation: Pay close attention to the color changes, precipitate formation, and other visual cues during experiments. These subtle changes are often the key to unlocking the properties of aldehydes and ketones.
Properties Of Aldehydes And Ketones Lab Report
A Final Note: Embracing the Enchantment of Chemistry
Our journey through the world of aldehydes and ketones has unraveled their intricate structures, diverse properties, and profound impact on our lives. From the aromas that tantalize our senses to the molecules that underpin life itself, these compounds are a testament to the versatility and enchantment of chemistry.
This lab report is not merely a record of experiments; it is an invitation to explore further, to delve deeper into the fascinating world of organic chemistry, and to appreciate the profound ways in which the seemingly simple molecules around us shape our world.






