As a child, I was fascinated by the idea of the elements – those fundamental building blocks that make up everything around us. The periodic table, with its rows and columns, seemed like a magical map leading to a world of atoms and molecules. This fascination hasn’t faded, and exploring the first thirty elements is a great way to understand the very nature of the universe.

Image: www.pinterest.com
From the lightest element, hydrogen, to the reactive chlorine, this initial section of the periodic table holds a wealth of information. We will embark on a journey to learn about their properties, origins, and how they contribute to our daily lives. This comprehensive guide aims to provide you with a clear and nuanced understanding of the first thirty elements.
A Foundation of the Universe: The First Thirty Elements
The Essence of Simplicity
The first thirty elements, often referred to as the “light elements,” embody a core set of chemical principles. They make up the vast majority of matter in the universe, including stars, planets, and the very air we breathe. Understanding these elements is fundamental to grasping the basic building blocks of all matter.
These fundamental building blocks are defined by their atomic numbers, which correspond to the number of protons in their nucleus. As we move across the periodic table, from hydrogen with one proton to zinc with thirty, we observe a gradual increase in the number of protons and electrons in each atom. This increase in complexity determines their unique chemical and physical properties.
An Evolutionary Journey
The early universe was dominated by hydrogen and helium, the simplest elements. Over millions of years, these elements fused together in stars to create heavier elements through a process called nuclear fusion. This process is responsible for the formation of the remaining elements, including those found within the first thirty.
Elements like carbon, oxygen, and nitrogen are essential for life as we know it. Without them, the complex molecules that make up plants, animals, and even our own bodies would not exist. These elements create a natural progression, building upon each other in a fascinating evolutionary process that shapes the universe.
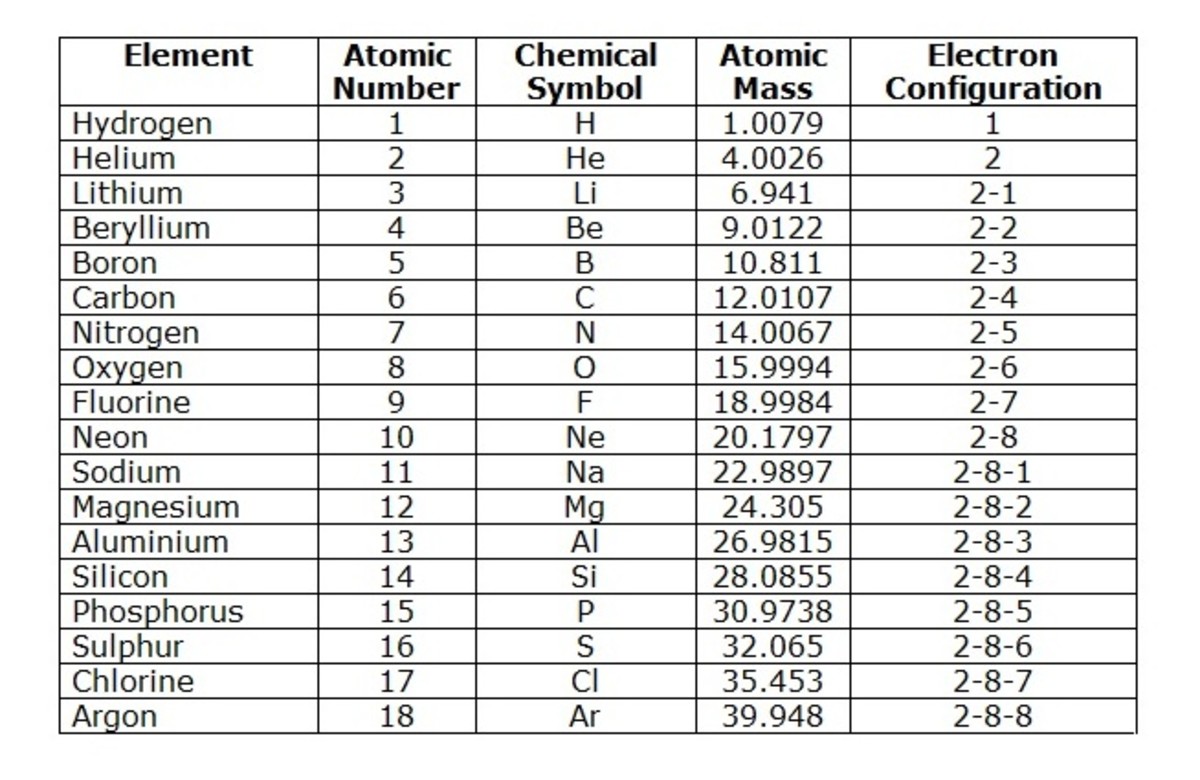
Image: ar.inspiredpencil.com
A Glimpse of the Periodic Table
To comprehend the first thirty elements, it’s essential to understand the structure of the periodic table. The table is arranged by atomic number, with elements with similar properties grouped together in columns known as “groups” or “families.” The rows, called “periods,” reflect the electron configuration of the elements. These configurations influence chemical reactivity and bonding behavior.
The first period comprises just two elements: hydrogen and helium. Hydrogen is the most abundant element in the universe, while helium is a noble gas, meaning it is chemically inert. The second period houses eight elements, including lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, and neon. This period gives rise to a fundamental shift in properties, as we move from the extremely reactive alkali metals (like lithium) to the highly electronegative halogens (like fluorine).
From Alkali Metals to Transition Metals
The third period continues the pattern, incorporating sodium, magnesium, aluminum, silicon, phosphorus, sulfur, chlorine, and argon. Sodium is a key electrolyte in our bodies, while silicon forms the backbone of computer chips. Here, we encounter the transition metals for the first time, starting with scandium in the fourth period. These elements are known for their variable oxidation states and colorful compounds. They play important roles in various industries, from steel production to catalysis.
Each period onwards expands upon the previous, introducing new groups and new classes of elements. Each element, with its unique atomic structure and properties, contributes to the tapestry of the universe. Understanding the first thirty elements provides us with a foundation for unraveling the mysteries of the vast and complex world of chemistry.
The Evolution of Elements: From Big Bang to Stellar Furnaces
The process of element formation is a fascinating journey, starting from the very beginning of the universe. The Big Bang created an abundance of hydrogen and helium, the most fundamental elements. These elements coalesced into stars, where the intense pressure and heat ignited nuclear fusion, transforming them into heavier elements. In the heart of stars, elements like carbon, oxygen, and nitrogen were forged, paving the way for the emergence of life.
Over billions of years, stars have undergone their life cycles, eventually exploding as supernovas. These explosions scattered these newly created elements across the galaxy, enriching the interstellar medium and ultimately forming planets, including our own Earth. The elements found on Earth are the remnants of these ancient stellar processes, a testament to the cosmic origins of all matter.
Insights and Tips for Studying the First Thirty Elements
Learning about the first thirty elements can be an enriching experience, unlocking the secrets of the universe around us. Here are some tips to make your exploration more engaging and effective:
- Use Visual Aids: The periodic table is your ultimate guide. Familiarize yourself with its organization and consider using visual aids, such as color-coded charts or interactive models, to better understand the relationships between elements.
- Focus on Trends: Pay attention to the recurring patterns in the periodic table, such as electronegativity, atomic radius, and ionization energy. These trends offer valuable insights into the behavior of elements.
- Explore Applications: Connect the elements you are learning about to their real-world applications. Understanding how elements are used in everyday life can enhance your appreciation for their significance.
Remember, learning about the first thirty elements is a journey of discovery, not a race to memorize facts. Take your time, explore the properties of each element, and appreciate the beauty and complexity of the natural world.
Frequently Asked Questions
Q: What are the most important elements in the first thirty?
A: Hydrogen, oxygen, carbon, nitrogen, and calcium are among the most important elements in the first thirty. They are essential for life and play significant roles in various industries.
Q: How are the first thirty elements used in everyday life?
A: The first thirty elements are essential for everything from building materials to electronics to food production. For example, iron is used in steel, silicon in computer chips, and calcium in our bones and teeth.
Q: What are the challenges in understanding the first thirty elements?
A: The challenge lies in grasping the complex relationships between the atomic structure, chemical properties, and their applications. The periodic table provides a framework, but understanding the nuanced details of each element requires time and effort.
First Thirty Elements Of The Periodic Table
Conclusion
The first thirty elements are the foundation of the universe, shaping the stars, planets, and even life itself. Understanding their properties and how they interact with one another is key to unraveling the mysteries of chemistry and the origins of matter. From the simplest element, hydrogen, to the transition metals, each element tells a story that connects us to the cosmos.
Are you fascinated by the world of atoms and elements? Would you like to delve further into the captivating history and properties of the first thirty elements? Let us know in the comments below!






