Do you remember those colorful charts hanging in your high school chemistry class? The ones with all the strange symbols and numbers? They represented the periodic table, a vital tool for understanding the building blocks of the universe. Even if you were never a science whiz, the periodic table likely holds some familiarity. Today, we’ll embark on a journey through Period 2, the second horizontal row of the periodic table, specifically focusing on its elements and their unique symbols. This journey will reveal how these symbols relate to the elements’ names, their properties, and even their uses in our everyday lives.
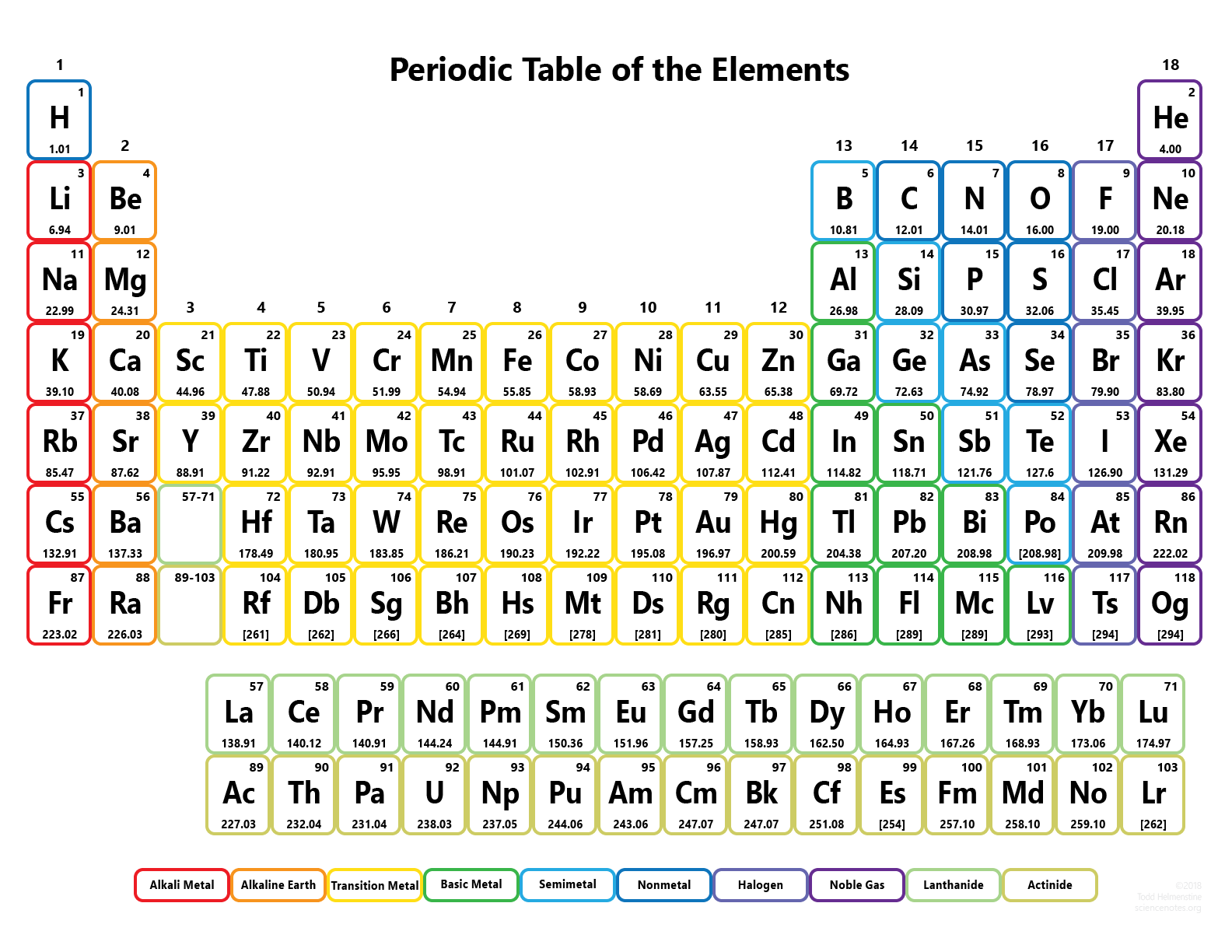
Image: www.aiophotoz.com
To grasp the significance of these symbols, imagine being a scientist trying to communicate findings across languages and cultures. How would you describe a specific element to someone who speaks a language entirely different from yours? This is where symbols become essential, providing a standardized, universal language for discussing elements – a language understood by scientists worldwide.
Exploring Period 2: Unveiling the Elements and Their Symbols
Period 2 is a fascinating row on the periodic table, containing elements that are crucial to life as we know it. It holds both nonmetals and metalloids, reflecting the diverse nature of this row. Each element in Period 2 has its own distinctive symbol, which captures its unique identity within the vast world of chemistry.
The Journey Begins: From Left to Right
Our journey through Period 2 starts with Lithium (Li), the first element in the row. Lithium’s symbol “Li” is derived directly from its Latin name, “lithium,” meaning “stone.” Right next to it is Beryllium (Be), with the symbol “Be.” Beryllium owes its symbol to its Latin name “beryllus,” which refers to the gemstone beryl. The next element, Boron (B), represented by the symbol “B,” gets its name from the borax mineral.
We then encounter Carbon (C), the backbone of life, which unsurprisingly has the symbol “C.” Nitrogen (N), with the symbol “N,” is essential for building amino acids and proteins. Its name derives from the Greek words “nitron” and “genes,” signifying “soda-producing.” Oxygen (O), symbolized by “O,” is the life-giving element we breathe. Its name comes from the Greek word “oxys,” meaning “acid.” Fluorine (F), denoted by “F,” is the most reactive element in Period 2 and is crucial for strengthening tooth enamel – hence its use in fluoride toothpaste. Fluorine takes its name from the “fluo” prefix, which refers to “to flow.”
The Last Element in Period 2: Neon’s Glowing Symbol
The last element in Period 2 is Neon (Ne) with the symbol “Ne.” Neon is known for its beautiful reddish-orange glow when excited by electricity – a phenomenon familiar from neon signs. While the element’s name comes from the Greek word “neos,” meaning “new,” it’s actually a very common element in the universe.
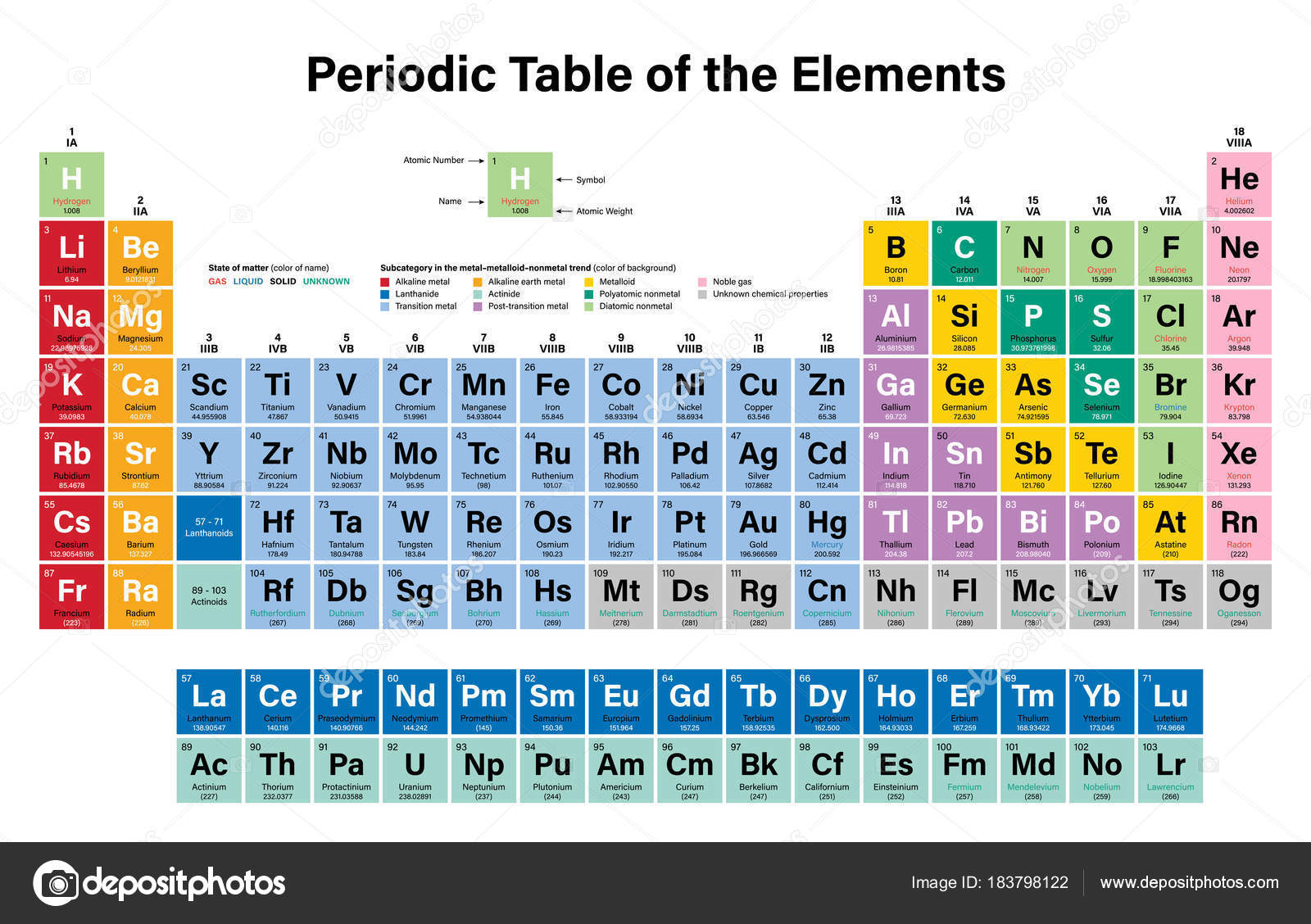
Image: mungfali.com
Understanding the Significance of Symbols
The symbols of the elements in Period 2 are more than just abbreviations. They are a shorthand for a vast amount of information about each element. They reveal the element’s history, its properties, and even its role in the world around us. By understanding these symbols, we unlock a greater understanding of the building blocks that make up our world.
Tips for Exploring the Periodic Table
Learning about the symbols of elements in Period 2 (or any other period) can be an exciting journey. Here are a few tips for making it engaging and enriching:
- Focus on the History: Research the history behind each element’s name and its symbol. Discover how the element was discovered and why it received a particular name.
- Connect with Applications: Explore the various uses of each element in everyday life. For example, how are Lithium batteries used in our phones and laptops, or how is Oxygen used in hospitals for medical purposes? Connecting the symbols to real-world applications can make learning more engaging.
- Visualize with Models: Utilize online tools or even create your own model of Period 2. This visual representation can help solidify your understanding of the elements and their symbols.
Beyond these general tips, remember to approach this exploration with an inquisitive mind. Ask questions, seek answers, and let curiosity be your guide. As you delve deeper into the world of chemistry, you’ll realize that the symbols of the elements are not just random letters but a language that unlocks a vast universe of knowledge.
Frequently Asked Questions (FAQ) about Period 2
Q: What is the most reactive element in Period 2?
A: Fluorine (F) is the most reactive element in Period 2. This reactivity stems from its high electronegativity, a measure of its ability to attract electrons.
Q: What element in Period 2 is found in the human body?
A: Several elements in Period 2 are found in the human body, with Oxygen (O), Carbon (C), and Nitrogen (N) being the most abundant.
Q: Can I find Period 2 elements in my home?
A: Absolutely! Oxygen (O) is in the air you breathe, Carbon (C) is found in the food you eat (and your furniture!), and Neon (Ne) signs brighten streets in many cities.
Q: What makes Period 2 a special period?
A: Period 2 contains a diverse range of elements, including nonmetals, metalloids, and the elements that form the basis of life. It also features the highly reactive element Fluorine and the inert element Neon.
List The Symbols Of The Elements In Period 2
Conclusion: The Symbols of Period 2 – A Gateway to Knowledge
The symbols of elements in Period 2 are not just a set of letters – they represent a journey through the building blocks of our world. From the very reactive Fluorine to the inert Neon, these elements shape our lives in ways we may not even realize. As you learn more about these symbols and the elements they represent, you’ll discover the beauty and complexity of the world around us. Are you ready to explore the fascinating world of Period 2 and unlock its incredible stories?






